Ensuring compliance with FDA complaint-handling regulations requires a well-defined QA framework that evaluates every aspect of customer interactions. MaestroQA supports manufacturers with a comprehensive QA program that monitors compliance, evaluates performance, and generates audit-ready reports.
How the QA Framework Works
Accurate Complaint Identification: Ensure proper complaint classification, escalation, and detailed documentation.
Comprehensive Technical Support Evaluation: Monitor troubleshooting accuracy, procedural adherence, and service record completeness.
Action-Oriented Communication Standards: Maintain clear, empathetic, and well-documented communication.
Core Evaluation Metrics
Comprehensive complaint management involves tracking specific metrics tied to complaint identification, technical support accuracy, and communication standards. These metrics ensure compliance with FDA Quality System Regulations while maintaining high operational performance.

Ensure accurate complaint classification, timely escalation, and detailed reporting.
Metrics Evaluated:
- Correct classification of complaints vs. general inquiries
- Complete data capture
- Proper escalation of reportable events
- Timely routing to the complaint-handling team

Deliver accurate, well-documented technical support in compliance with validated procedures.
Metrics Evaluated:
- Correct troubleshooting and technical guidance
- Documentation of issue resolution and service records
- Adherence to technical procedures
- Proper escalation for unresolved issues

Ensure professional, empathetic, and clear communication with customers.
Metrics Evaluated:
- Clear, accurate, and professional messaging
- Correct and complete issue descriptions
- Proper customer follow-up and next-step expectations
- Documentation of all correspondence
Quality Monitoring Process
Effective complaint management requires ongoing evaluation processes to ensure agents consistently meet compliance requirements. MaestroQA streamlines these evaluations with targeted reviews and compliance audits at every critical stage.
Routine Evaluations
100% Review of Identified Complaints: Ensure complete complaint reviews for regulatory compliance.
Random Sampling of Non-Complaint Interactions: Review 10% of other customer interactions for missed issues.
Agent Evaluations: Evaluate at least five agents weekly on complaint resolution accuracy, compliance, and service quality.
Targeted Audits During Risk Periods: Conduct focused evaluations during product launches or high-risk periods.
Performance Management
Real-Time Feedback: Deliver immediate feedback on critical compliance issues.
Weekly Agent Performance Reviews: Evaluate agent performance with regular coaching sessions.
Monthly Team Analysis: Conduct data-driven team reviews to address systemic issues.
Quarterly Compliance Training Updates: Ensure staff stays current on evolving regulatory requirements through targeted training.
Corrective Actions
Individual Level
- Immediate coaching and regulatory compliance training for violations.
- Customized performance improvement plans with documented follow-up.
System Level:
- Process adjustment recommendations based on findings.
- Updated training programs and procedural enhancements.
- Technology updates for automation and efficiency improvements.
Custom Scorecard for FDA Complaint Evaluations
Custom scorecards provide a scalable way to evaluate agent performance while ensuring that FDA-specific requirements are consistently monitored. They enable precise, criteria-driven scoring for streamlined compliance.
What are QA Scorecards?
A QA scorecard, also known as a rubric, is a structured scoring guide used to evaluate agent performance against specific standards. By creating scorecards tailored to FDA Regulations, businesses can:
- Ensure complaints are classified and escalated correctly.
- Adhere to troubleshooting and service documentation protocols.
- Maintain accurate service records for FDA inspections.
- Deliver empathetic, action-driven responses.
With a properly set up QA scorecard, businesses can turn compliance requirements into actionable insights that improve outcomes for both agents and customers.
Example Scorecard Criteria
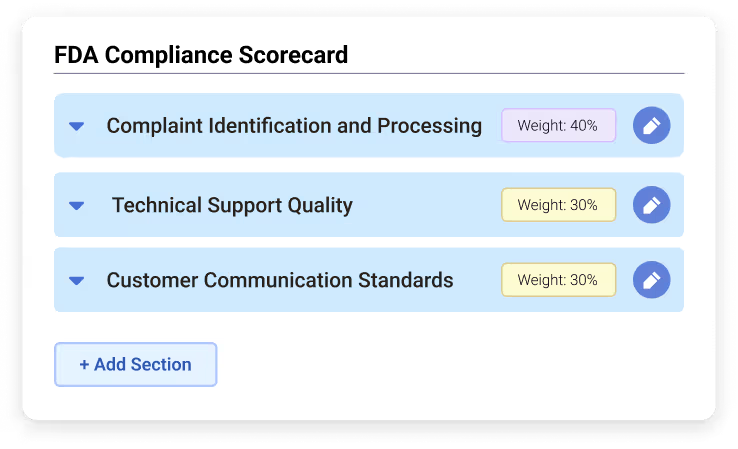
Section One: Complaint Identification and Processing
- Accurate identification of complaints vs. general inquiries
- Complete capture of required complaint information:
- Customer contact details
- Device identifiers (lot/serial numbers)
- Detailed description of the reported issue
- Date and time of receipt
- Proper escalation of potential reportable events
- Timely routing to complaint handling team
Section Two: Technical Support
- Accuracy of technical guidance provided
- Proper documentation of troubleshooting steps
- Adherence to validated technical procedures
- Completeness of interaction documentation
- Appropriate escalation of technical issues
Section Three: Communication Quality
- Professional and clear communication
- Complete and accurate information delivery
- Appropriate empathy and urgency
- Clear next steps and expectations setting
- Proper documentation of all customer correspondence
Key Highlights
Complaint Identification: Ensure all product issues are classified and escalated according to FDA guidelines with fully documented complaint details.
Technical Support Quality: Maintain thorough records of troubleshooting steps, issue resolutions, and service documentation for FDA inspections.
Customer Communication Excellence: Ensure all customer interactions are clear, empathetic, and professionally documented, with next-step resolutions clearly outlined.
Scorecard Configuration

- Binary scoring for regulatory requirements (pass/fail)
- Weighted scoring for quality elements
- Auto-fail triggers for missed complaints or safety issues

- Accuracy measurement against validated procedures
- Documentation completeness checks
- Resolution verification metrics

- Customer experience metrics
- Professionalism standards
- Documentation quality assessment
Tools That Power the QA Framework
A strong QA framework requires robust tools that track performance, evaluate compliance, and generate audit-ready reports. MaestroQA’s platform offers essential tools that make compliance more efficient and transparent.
Calibration for Compliance Accuracy
Achieving consistent compliance scoring requires regular calibration sessions. MaestroQA's calibration process ensures alignment between QA teams, regulatory teams, and senior management.
Weekly Calibration Sessions: QA teams calibrate scores to maintain evaluation accuracy.
Monthly Cross-Functional Reviews: Collaboration between QA and regulatory teams ensures shared understanding of compliance criteria.
Quarterly Senior Management Audits: Leadership teams validate evaluation outcomes, ensuring continuous improvement and compliance integrity.
Training Integration: Update training programs based on evaluation findings and document competency assessments and ongoing certification programs.
Conclusion
A strong QA framework is essential for ensuring regulatory compliance, minimizing risk, and improving operational efficiency. MaestroQA empowers manufacturers to achieve these goals with an integrated, scalable quality management system.
Learn how MaestroQA can strengthen your complaint-handling processes and ensure long-term FDA compliance. Contact us to learn more!
Legal Disclaimer: The information provided on this webpage is for informational purposes only and does not constitute legal advice. For specific advice regarding compliance with CCA or CDA regulations, please consult a qualified attorney.











































